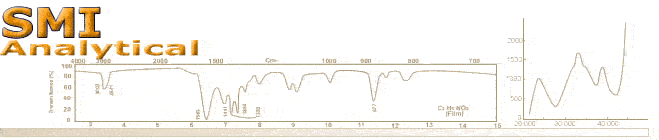
Unknown Chemical Substance Sampling
Chemical analysis affects most people, most of the time, throughout their life. Think of computers, agriculture, medicine, cars, toilet paper, fuel, toothpaste, medicine, food stuffs, clothes, detergents, roads, houses, our environment, etc
All have been or will be subjected to chemical analysis at some stage of their lives. The importance of chemical analysis and chemical testing can not be underestimated and has literally become a necessity since and before its invention.
Chemical analysis it rarely performed just for the sake of knowing, it is embedded and integrated into everyone's life whether or not the public know, care or worry about the fact. Chemical analysis and chemical testing does however ensure quality, productivity, safety, consistency, control and many other factors affecting everyday life.
Chemical testing can be divided into a few overlapping broad fields, to name most:
- Inorganic
- Organic
- Qualitative
- Quantitative
- Biological
- Wet or "classical"
- Instrumental
Samples types could literally be anything, i.e. wood, soil, water, plastic, gas, cloth, liver tissue, etc
. When analyzing a substance the normal questions that come up and normally in this order are:
- What are the samples composed of (qualitative analysis) and how much of each substance is present (quantitative analysis).
Chemical analysis and
chemical testing can take from 1 minute to a few months depending on type and depth required.
Another important question is the accuracy required for an analysis or chemical test. This often affects time and cost.
For example: % copper in ore. The result could be reported as 15 +-1% or 15.21 +-0.05% depending on various analysis factors and the required accuracy or rather, the confidence limits. The term
"confidence limit" should be used because if the actual concentration of the copper is 15.03 +-0.02%, then the former concentration reported (15 +-1%) would be, technically, more "accurate" as accuracy refers to the agreement of the actual or accepted value. But, which result would you rather accept or report. Normally results are reported without these confidence limits.
Chemical analysis is never "absolute" and always limited to statistical variations and statistics is a huge part of chemical analysis.
For chemical analysis or chemical test to be absolute, one would need to be able to "see" each and every atom. As an example there are 602 300 000 000 000 000 000 000 atoms in only 12g of pure Carbon, count them and also count all the other elements present in sample .Clearly this is Impossible, and one can see why statistics play a vital role in chemical testing.
One more critical question is the
detection limit required.
A substance (be it compound or element) can never be said not to exist in a sample. It can only be reported as less than a certain detection limit which is limited by the instrument, method or technique.
Ensuring accurate chemical analysis or chemical testing is only part of the puzzle. Interpretation of results is just as important. Clearly, it's one thing to just know the concentrations of certain chemicals in various fertilizer samples but another to know how to utilize the data and to blend or mix them into workable, a to z customized, hydroponic nutrient solution.
SMI Analytical can assist you with both Chemical Analysis and Interpretation of chemical data obtained in chemical tests.