|
CLAY ANALYSIS / CLAY IDENTIFICATION
|
MENU
CONTACT DETAILS
|
|
|
|
|
|
Clay Analysis / Clay Identification
SMI Analytical has developed various techniques to assist you with clay analysis.
These range from X-Ray diffraction (XRD) to Scanning Electron Microscopy (SEM).
Clay mineral identification is not an easy task, clay minerals are similar and are often interstratified with each other. At SMI Analytical we have adopted and developed techniques to aid in clay mineral investigation .These include: Heat treatment at 400 and 550 Celsius, Glycolation and Forced Ion exchange .If due diligence is shown not only can clays be identified as to there individual components, they can also be quantified by Rietveld Methodology. This is a major plus for many industries, where exact mineral quantification of clays is important and takes the guess work out clay based formulations.
Clay Analysis and Clay Mineral Quantification is extremely important to all Ceramic Industries to formulate there raw clay bodies and look at resulting fired products .Clay analysis takes the guess work out of formulating clay recipes for firing.
Road and Construction Industries have to do clay analysis as the Smectite family of clays, swell dramatically when exposed to water. This expansion and contraction of the soil can cause extensive damage to buildings and their foundations, roads, and other structures.
SMI Analytical has helped many international and local pharmaceutical and cosmetic companies with clay analysis. Clay analysis is important to establish that contaminants are not present eg)Pesticides, Herbicides and other Hazardous Chemicals. This is particularly important when clays are used as face packs and other cosmetic applications, as well as ensuring that their clay based recipes remain consistent.
Clay Mineralogy
Below is an introduction to clay mineralogy
- Clay minerals are an important group of minerals because they are among the most common products of chemical weathering, and thus are the main constituents of the fine-grained sedimentary rocks called mud rocks (including mudstones, claystones, and shale's). In fact clay minerals make up about 40% of the minerals in sedimentary rocks. In addition, clay minerals are the main constituent of soils. Understanding of clay minerals is also important from an engineering point of view, as some minerals expand significantly when exposed to water.
- Clay minerals are used extensively in the ceramics industry and are thus important economic minerals.
- Based on their structures and chemical compositions, the clay minerals can be divided in to four main classes: (Some Clay Scientists refer to 2 or 3 classes for clays:Chlorite is sometimes referred to as a 2:1 layer not a 2:1:1 and Illites/Micas are sometimes grouped with Smectites)
Kandites 1:1 layered clays eg)Kaolinite
Smectites 2:1 layered clays eg)Montmirrilonite
Illites/Micas 2:1 layered clays eg)Muscovite
Chlorites 2:1:1 layered clays eg)Clinochlore
The Kandites
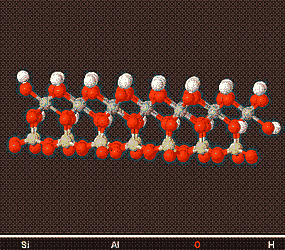
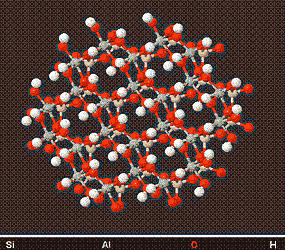
Above is a model of a Kaolin Crystal viewed from different angles, showing its structure. Kaolin minerals form plate like structures which are visible by Electron Microscopy.
The Kaolinite family of Clay Minerals are formed by weathering or hydrothermal alteration of aluminosilicate minerals. Because of there chemical weathering resistance and non-swelling nature they are the clay of choice in ceramics and porcelain products.
The Smectites
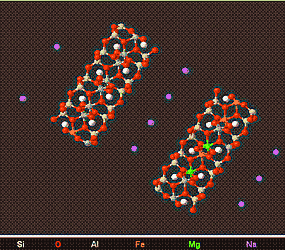
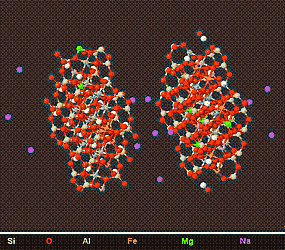
Above is a model of a Smectite Crystal viewed from different angles. The Interlayer space can clearly be observed and this accounts for the swelling nature of smectites.
Smectite rich soils can undergo as much as a 40% volume change due to water being absorbed and desorbed in Interlayer space. The force behind this swelling is immense,and can raise buildings and structures by a few centimetre's causing cracks and other problems. Due to there swelling nature, Smectites are useful as a drilling mud (to keep drill holes open), and to plug leaks in soil, rocks, and dams.
The Illites/Micas
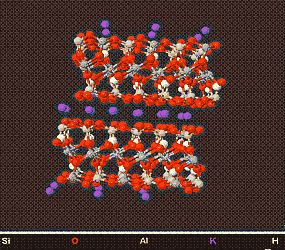
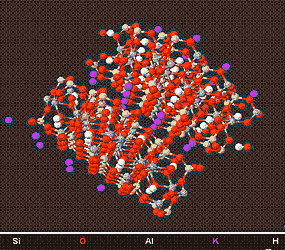
Above is a model of a Muscovite Crystal (a good example of an Illitic/Micaceous Mineral) viewed from different angles, showing its structure.
Illitic/Micaceous Clays or a major component of Ball clay, and as such have a major use in the ceramic and clay brick industry. Although Illites have a interlayer space, the attraction between the 2 plates is extremely strong, and prevents Illites/Micas from swelling, when in contact with water .Many micaceous materials have a sheen and are used in metallic paints.
The Chlorites
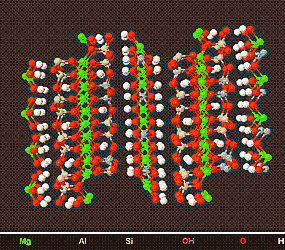
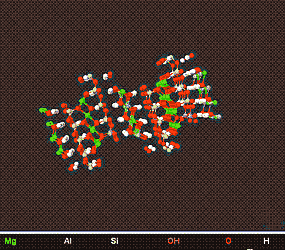
Above is a model of a Typical Chlorite Crystal viewed from different angles.
Chlroritic clay is mainly associated with Illitic/Micaceous Clay and as such is used in the ceramic industry .It is easily weathered into other clay minerals,and as such is the least occuring clay mineral .In fact deposits of predominantly chlorite clay have yet to be discovered.
Pictures of Kaolinite and Illite
Below are Scanning Electron Microscope Pictures of Kaolinite Interstratified with Illite. Note the platelets of Kaolin are easily observed.
XRD Scan Comparison Fired Clay
X-Ray diffraction is a powerful tool in all mineralogical work,Below is an example of comparative XRD scans showing the change in Raw clay compared to Fired Tile. Clay was fired at 1150 Celsius for 25 minutes.
Notice the clay peaks(Illite at 8.9 ,and Kaolinite at 12.3 Degrees 2 Theta have disappeared and a new peak at 16.5
Degrees 2 Theta has formed.
This new peak is the mineral Mullite. Mullite is what forms when clays are fired and is the mineral we associate with ceramics.
|
|
|
|
Disclaimer: The liability of SMI ANALYTICAL is limited to the cost of analysis. SMI ANALYTICAL indemnifies
itself from any legal action which may be instituted against it due to supplied data. SMI ANALYTICAL accepts
no responsibility whatsoever for any results released, however used. No part of this document may reproduced
in part or in full unless permission from SMI ANALYTICAL is granted in writing. This document may not be altered
in any way whatsoever and is printed without correction.
|
Analytical Chemistry Links
Some Background about X-Ray Diffraction
University of Cambridge - Analytical Chemistry Journals
The Clay Minerals Society - Online Courses, Lectures, and Labs
Sheffield Chemdex is the directory of analytical chemistry on the World-Wide Web
|
|
Analytical Lab
SMI Analytical Laboratory Services / Chemical Laboratory Services specializes in quantitative X-Ray diffraction
clay analysis sampling: X-Ray diffraction of Clay, scanning electron microscopy clay minerals,clay testing, clay chemical properties, clay physical properties, swelling clay identification, clay quantification, XRD, SEM, clay pollution, clay testing, clay mining, clay quarry, clay for tiles, ceramic clay, ceramic properties, clay properties, clay lab
clay analysis, sampling, scanning electron microscopy, x-ray diffraction, SEM, XRD, ceramic industries, tile manufacturing, brick manufacturing, quantitative mineral analysis, clay phase, clay minerals, montmirrilonite, smectite, illite, mica, kaolin, kaolinite, mullite, tile bodies, chlorite, clay mineral analysis, healing clay, mud packs, clay impurities, clay contaminants, clay face packs, cosmetic clay, pharmaceutical clay, laboratory,
site:, =, '.co.za', SA, RSA, za, 'loc:za'
|
|